Our Technology
Our SpyTag/SpyCatcher protein superglue enables us to avoid many of the challenges of binding antigens to virus-like particles
Vaccines rely on the body’s immune system recognizing foreign proteins called antigens and responding to them by creating antibodies to fight against infection.
Spy technology can be incorporated with different platforms to create a plug-and-play toolbox for vaccine development with several advantages.
SPYVLP Platform
However, binding antigens to the coat proteins of VLPs in a controlled and reliable way using conventional technology is highly complex. Directly fusing antigens to VLPs often damages the shape of the antigens or VLPs or both, rendering the vaccine ineffective. The alternative of chemically binding the antigens to the VLP is a complex and time-consuming process that does not allow scientists to control the quantity or orientation of antigens that attach to the VLP. This can also compromise the efficacy of the vaccine.
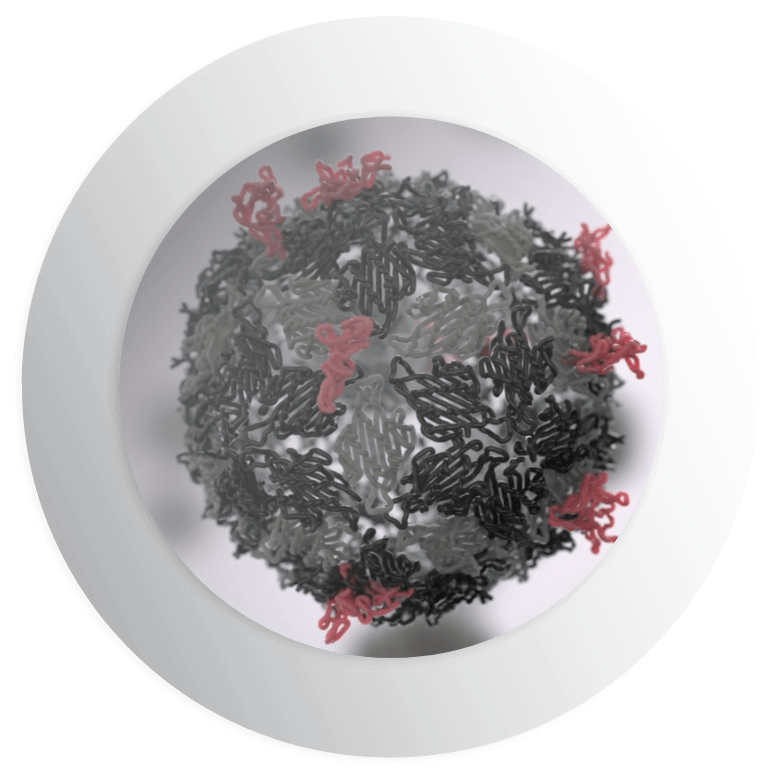
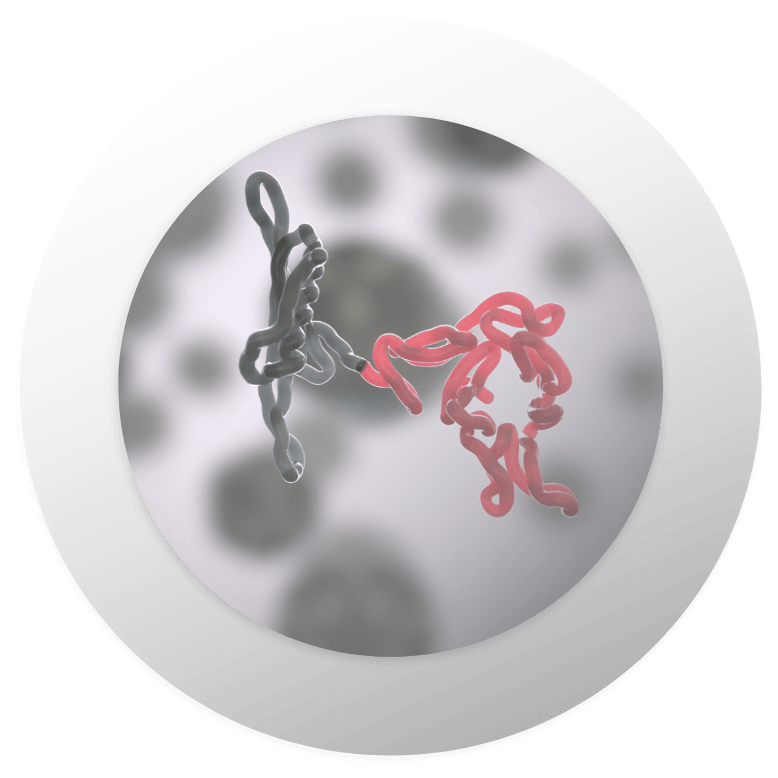
Our proprietary SpyTag/SpyCatcher protein superglue enables us to circumvent these issues. Our unique technology allows us to split a protein from the common bacterium, Streptococcus pyogenes, into two parts: the SpyTag peptide that can be bound to antigens and its partner protein SpyCatcher that binds to the VLP. The two pieces of the original SpyTag/SpyCatcher protein then bind back together in a spontaneous conjugation forming an unbreakable covalent bond.
The process is rapid, efficient, irreversible and extremely versatile. It allows for specific assembly of antigens on VLPs to generate an optimal immune response and can be applied to a wide range of infectious viral, bacterial and parasitical diseases and potentially to cancer and allergies. Both SpyTag and SpyCatcher can be made in any cell type.
Armed with this breakthrough technology we are able to generate a wide range of vaccines candidates.
SPYVECTOR Platform

SPYVECTOR is a platform based on recombinant adenovirus. The platform enables easy and efficient covalent decoration of the surface of the adenovirus with pathogen antigens in addition to genetically encoding the antigen.
The platform increases the quantity of antibodies induced by decorating the adenovirus with the antigen while maintaining the T cell response to the encoded antigen. This display can shield and avoid anti-vector immunity, thereby increasing the possibility of using the same vector again and again. SpyBiotech is exploring both infectious disease and other applications for SPYVECTOR.
Broader applications of our technology
SpyBiotech’s proprietary technology has an exceptionally broad range of applications in vaccine development. The simple, efficient and irreversible reaction between the peptide SpyTag and its protein partner SpyCatcher provides unique opportunities to assemble vaccines against viruses, bacteria and parasites.
Spy-based immunotherapy has also shown promise against cancer and chronic diseases. In the last few years, work at SpyBiotech and by academic groups around the world has generated important data on these diverse targets and different immunization platforms.
Viral Diseases
In the context of viruses, SpyTag has been used for vaccine assembly for global challenges that have resisted standard vaccine approaches over decades, as well as for newly emerging viral outbreaks.
SpyBiotech is leading the development of a vaccine against cytomegalovirus (CMV), which is present throughout the world and is a major cause of deafness, blindness and neurological disabilities in babies.
SpyTag has been employed by leading groups at the NIH and Caltech towards a vaccine for HIV(1,2).
SpyTag-based platforms were used to generate vaccine candidates for vector-transmitted viruses, such as Tick-borne encephalitis3 and Zika Virus4.
Most work has used Spy technology prophylactically, constructing vaccines, but recent work has shown that Spy-based nanoparticle assembly also has therapeutic potential, towards clearance of Hepatitis B Virus5.
As soon as the SARS-CoV-2/Covid-19 outbreak started, academics began to use SpyTag-technology to move towards clinical testing of a vaccine.
Bacterial Diseases
The bacterium E. coli is a great friend to molecular biologists in laboratory experiments, but certain strains of E. coli cause severe and life-threatening infections. Spy-based VLPs have induced effective antibodies in animal models to block the action of a toxin from dangerous strains of E. coli6.
Spy-technology can be used on virus-like particles (VLPs) but also many other vaccine platforms: SpyTag facilitated the decoration of bacterial outer membrane vesicles (OMVs) to induce immune responses against Streptococcus pneumoniae, a globally significant pathogen which can cause death through pneumonia, meningitis or sepsis7.
Parasite Diseases
The academic founders of SpyBiotech have long worked towards the development of a malaria vaccine. Malaria is one of the hardest challenges in vaccine development because of the complexity of the parasite’s lifecycle, its genetic variability and the high level of antibody needed for protection. The earliest work on SpyTag using VLPs showed its potential for potent antibody induction against malaria, demonstrating responses to different life-cycle stages (pre-erythrocytic, blood-stage and transmission-blocking)(8-10).
Work against malaria showed how SpyTag-based vaccines induced higher quality antibodies in terms of their potential to protect from transmission of the disease11.
Cancer & Chronic Disease
It is now clear that the immune system is key to fighting cancer. Recent advances have shown how taking the brakes off the immune system (using checkpoint-blocking antibodies) can reveal potent tumor-killing T cell responses. Also, identifying patient-specific genetic changes (a personalized medicine approach) can allow stronger immunity than responses against more general features of cancer.
Spy technology has shown its versatility in pre-clinical testing against large proteins expressed on the cancer cell-surface (e.g. HER2)12, cancer-related peptides8 , tumor-specific neoepitopes13, or immune checkpoint proteins9. Such immune activation has led to both protective antibody and cytotoxic T lymphocyte responses(12,13).
Spy-based targeting is also being developed against virus-induced cancer, caused by the Human Papilloma Virus (HPV)14. In the area of chronic disease, Spy technology has shown promise in pre-clinical models for immunotherapy of allergy15.
Veterinary Disease
Spy Biotech has exclusive rights from Oxford to generate veterinary vaccines utilizing this technology. Billions of animals around the world receive vaccines, including pets, horses and farmed animals. Many of these vaccines depend on inactivated or attenuated forms of the disease-causing organisms. This classic approach requires large-scale culture of pathogens that people would rather eradicate from the planet, as well as running the risk of attenuated pathogens regaining virulence.
The EU-funded Zoonoses and Anticipation Preparedness Initiative (ZAPI) is developing vaccine approaches for major veterinary and zoonotic challenges: Rift Valley Fever, affecting humans and livestock; Schmallenberg virus, which affects livestock; and MERS, a coronavirus from camels that caused a global outbreak in humans in 2012 (See link).
ZAPI has focused on the use of Spy-VLPs to enhance the future development of veterinary vaccines, to improve both the efficacy and speed of response.
Spy technology has shown its versatility in pre-clinical testing against large proteins expressed on the cancer cell-surface (e.g. HER2)12, cancer-related peptides8 , tumor-specific neoepitopes13, or immune checkpoint proteins9. Such immune activation has led to both protective antibody and cytotoxic T lymphocyte responses(12,13).
Spy-based targeting is also being developed against virus-induced cancer, caused by the Human Papilloma Virus (HPV)14. In the area of chronic disease, Spy technology has shown promise in pre-clinical models for immunotherapy of allergy15.
References
- A. Escolano, H. B. Gristick, M. E. Abernathy, J. Merkenschlager, R. Gautam, T. Y. Oliveira, J. Pai, A. P. West, C. O. Barnes, A. A. Cohen, H. Q. Wang, J. Golijanin, D. Yost, J. R. Keeffe, Z. J. Wang, P. Zhao, K. H. Yao, J. Bauer, L. Nogueira, H. Gao, A. V. Voll, D. C. Montefiori, M. S. Seaman, A. Gazumyan, M. Silva, A. T. McGuire, L. Stamatatos, D. J. Irvine, L. Wells, M. A. Martin, P. J. Bjorkman and M. C. Nussenzweig, Nature, 2019, 570, 468.
- S. J. Krebs, Y. D. Kwon, C. A. Schramm, W. H. Law, G. Donofrio, K. H. Zhou, S. Gift, V. Dussupt, I. S. Georgiev, S. Schatzle, J. R. McDaniel, Y. T. Lai, M. Sastry, B. S. Zhang, M. C. Jarosinski, A. Ransier, A. L. Chenine, M. Asokan, R. T. Bailer, M. Bose, A. Cagigi, E. M. Cale, G. Y. Chuang, S. Darko, J. I. Driscoll, A. Druz, J. Gorman, F. Laboune, M. K. Louder, K. McKee, L. Mendez, M. A. Moody, A. M. O’Sullivan, C. Owen, D. J. Peng, R. Rawi, E. Sanders-Buell, C. H. Shen, A. R. Shiakolas, T. Stephens, Y. Tsybovsky, C. Tucker, R. Verardi, K. Y. Wang, J. Zhou, T. Q. Zhou, G. Georgiou, S. M. Alam, B. F. Haynes, M. Rolland, G. R. Matyas, V. R. Polonis, A. B. McDermott, D. C. Douek, L. Shapiro, S. Tovanabutra, N. L. Michael, J. R. Mascola, M. L. Robb, P. D. Kwong and N. A. Doria-Rose, Immunity, 2019, 50, 677. View More References
- Z. Liu, H. Zhou, W. Wang, W. Tan, Y. X. Fu and M. Zhu, Scientific reports, 2014, 4, 7266.
- W. A. De Jongh, T. M. M. Sogaard and T. D. Jorgensen, US Patent and Trademark Office, 2017, US20190358313.
- W. Wang, X. Zhou, Y. Bian, S. Wang, Q. Chai, Z. Guo, Z. Wang, P. Zhu, H. Peng, X. Yan, W. Li, Y. X. Fu and M. Zhu, Nat Nanotechnol, 2020, DOI: 10.1038/s41565-020-0648-y.
- M. L. Govasli, Y. Diaz and P. Puntervoll, Vaccine, 2019, 37, 6405-6414.
- H. B. van den Berg van Saparoea, D. Houben, M. I. de Jonge, W. S. P. Jong and J. Luirink, Applied and environmental microbiology, 2018, 84, e02567.
- K. D. Brune, D. B. Leneghan, I. J. Brian, A. S. Ishizuka, M. F. Bachmann, S. J. Draper, S. Biswas and M. Howarth, Scientific reports, 2016, 6, 19234.
- S. Thrane, C. M. Janitzek, S. Matondo, M. Resende, T. Gustavsson, W. A. de Jongh, S. Clemmensen, W. Roeffen, M. van de Vegte-Bolmer, G. J. van Gemert, R. Sauerwein, J. T. Schiller, M. A. Nielsen, T. G. Theander, A. Salanti and A. F. Sander, Journal of nanobiotechnology, 2016, 14, 30.
- M. Sungwa, T. Susan, J. C. Mikkel, K. R. Adolph, M. S. Boniface, T. T. Grundtvig, S. Ali, N. M. Agertoug and S. A. Frederik, Afr. Health Sci., 2017, 17, 373-381.
- D. B. Leneghan, K. Miura, I. J. Taylor, Y. Li, J. Jin, K. D. Brune, M. F. Bachmann, M. Howarth, C. A. Long and S. Biswas, Scientific reports, 2017, 7, 3811.
- A. Palladini, S. Thrane, C. M. Janitzek, J. Pihl, S. B. Clemmensen, W. A. de Jongh, T. M. Clausen, G. Nicoletti, L. Landuzzi, M. L. Penichet, T. Balboni, M. L. Ianzano, V. Giusti, T. G. Theander, M. A. Nielsen, A. Salanti, P. L. Lollini, P. Nanni and A. F. Sander, Oncoimmunology, 2018, 7, e1408749.
- W. Wang, Z. Liu, X. Zhou, Z. Guo, J. Zhang, P. Zhu, S. Yao and M. Zhu, Nanomedicine, 2019, 16, 69-78.
- Z. Liu, H. Zhou, W. Wang, Y. X. Fu and M. Zhu, Oncoimmunology, 2016, 5, e1147641.
- T. Soongrung, K. Mongkorntanyatip, T. Peepim, S. Jitthamstaporn, P. Pitakpolrat, T. Kaewamatawong, C. M. Janitzek, S. Thrane, A. F. Sander and A. Jacquet, Allergy, 2019, DOI: 10.1111/all.14096.